Patrick Guertler and Ulrich Busch
Global Situation
The global cultivation of genetically modified (GM) plants has increased since commercialisation in 1996. The area increased from 1.7 MHa in 1996 to more than 170 MHa in 2012 (Figure 1). The main GM crops are soybean (80.7 MHa), corn (55.1 MHa), cotton (24.3 MHa) and canola (9.2 MHa).
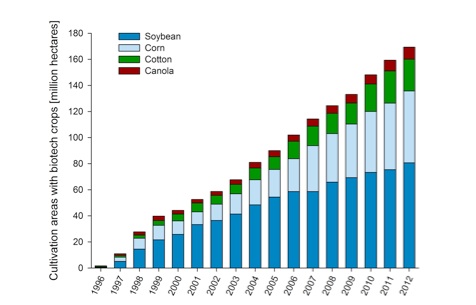
Figure 1: Global cultivation of genetically modified plants from 1996 till 2012. [Patrick Guertler, adapted from James (2012)]
In 2012, commercial cultivation took place in 28 countries worldwide, of which 20 were developing countries (Figure 2). The main global players in regard to cultivation of GM plants are the USA (69.5 MHa), Brazil (36.6 MHa), Argentina (23.9) MHa, Canada (11.6 million MHa and India (10.8 MHa). These numbers are published yearly by the International Service for the Acquisition of Agri-Biotech Applications ISAAA (James 2012).
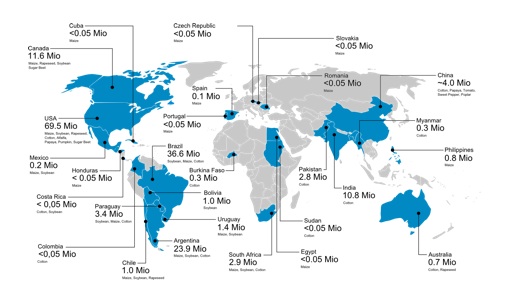
Figure 2: Countries growing genetically modified plants (Patrick Guertler, adapted from James 2012)
Situation in the European Union
In the European Union (EU), just five countries grew GM crops in 2012: Spain, Portugal, Slovakia, Romania and the Czech Republic. GM crops that enter the EU market must undergo a strict approval process. Placing on the market and labeling are regulated according to EU decrees 1829/2003 (EC 2003a) and 1830/2003 (EC 2003b). Products containing a GM crop above a GM content of 0.9 % must be labeled. Non-approved GM crops may not be placed on the EU market (zero tolerance policy). Currently, 58 GM crops are authorised as food / feed in the EU (as of June 2013).
Surveillance authorities like the Bavarian Health and Food Safety Authority are responsible for labeling control and feed / seed / food analysis (see http://www.lgl.bayern.de).
Detection of Genetically Modified Crops
Detection of GMO (genetically modified organism) can be accomplished by either detecting DNA or protein. For protein detection, the enzyme linked immunosorbent assay (ELISA) is the method of choice. ELISA is an antibody-based detection method, which is briefly described here: Protein-specific antibodies (capture antibodies) are immobilised in a 96-well plate. After that, a sample is added and proteins bind specifically to the antibodies. Subsequently, the detection antibodies are added, which are biotin, streptavidin and peroxidase labeled. These antibodies bind to another surface antigen of the protein. After adding a substrate, the enzyme peroxidase converts the colorless substrate into a colored substrate, which then can be measured photometrically. The conversion of colorless substrate into a colored substrate is proportional to the amount of bound protein (Figure 3). Therefore, the ELISA is also a quantitative detection method. However, reference material on protein level is not available.
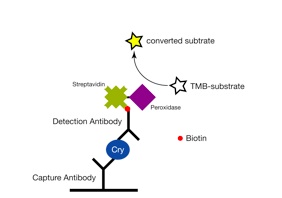
Figure 3: Detection of the Cry1Ab protein (GM corn event MON810) by way of an ELISA. (Guertler et al. 2009, Paul et al. 2008)
A rapid detection of proteins can also be conducted by using dip sticks. This detection system is very similar to ELISA. The antibody-protein-interaction and color change take place on a strip which is dipped into a sample-buffer-solution. For positive samples, bands will be visible on the strip, whereas one band misses in case of a negative sample. Quantification is not possible using dip sticks and this method also features a lower sensitivity compared to an ELISA. However, dip sticks can also be using in situ, e.g. on a field.
The lack of reference material on protein level is a major disadvantage of protein based GMO detection methods. And protein detection only proves the production of a certain protein, but does not allow distinction of several gm crop events. Therefore, GMO detection is mainly accomplished on the DNA level.
First of all, DNA needs to be isolated from sample material. By using detergents and enzymes, cells are lysed and released DNA can further be purified before used in subsequent analyses. DNA isolation is commonly performed manually or by using commercially available kits. Meanwhile, automated DNA extraction procedures are available (Guertler et al. 2013).
After DNA isolation, DNA needs to be amplified in order to be detectable. This is done by the polymerase chain reaction (PCR). The PCR is the method of choice for GMO detection and is comprised of three steps: a) double-stranded DNA is denatured at 94-95 °C; b) small DNA fragments (primers) bind specifically to the DNA fragment, which shall be detected; c) these primers are the starting point for the DNA polymerase, which completes the complementary DNA strand. Those three steps are repeated up to 45 times resulting in an exponential increase of DNA amount. The procedure is depicted in Figure 4.
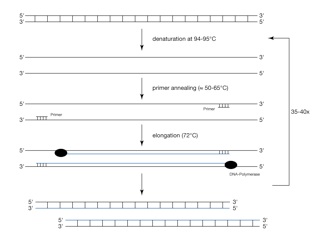
Figure 4: Schematic process of the polymerase chain reaction (PCR)
An improvement of the PCR is the quantitative real-time PCR (qPCR), by which DNA content can be quantified. The process of the qPCR is very similar to the conventional PCR as described above. However, DNA can be quantified, which is mainly achieved by using one of those two methods:
Method 1: A DNA binding dye like SYBR Green is added to the PCR reaction. The DNA-dye-complex absorbs blue light and emits green light, which can be detected. The more double-stranded DNA is present, the more dye binds to the DNA and the more green light is emitted (Figure 5). A major disadvantage is that these dyes also bind to primer dimers or to unwanted by-products, which then influences the fluorescence signal significantly.
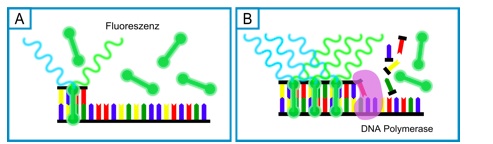
Figure 5: Scheme of a quantitative real-time PCR using the SYBR Green dye: A) SYBR Green binds unspecifically to double-stranded DNA; B) the more DNA is amplified, the more SYBR Green binds to double-stranded DNA and the more green light is emitted.
Method 2: A sequence-specific hydrolysis probe is added to the PCR reaction. This probe binds on the DNA strand between the forward and the reverse primer and is labelled with a reporter dye on its 5’ end and a quencher dye on its 3’ end. Due to the proximity of the reporter dye to the quencher dye, the fluorescence signal is quenched and therefore not detectable. In the PCR reaction, the polymerase extends the primers and hydrolyses the probe due to its exonuclease activity. As a result, this breaks the proximity of the reporter and quencher, allowing fluorescence of the reporter dye. The more DNA is amplified, the more probes are hydrolysed and the more light is emitted (Figure 6).
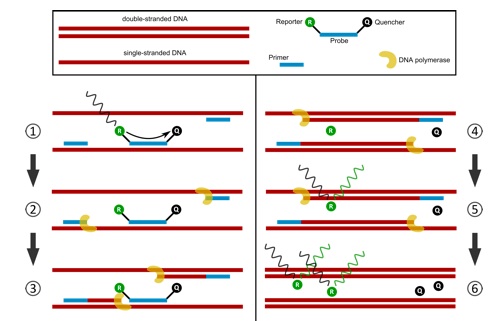
Figure 6: Scheme of a probe based quantitative real-time PCR assay.
Step 1: Primers and probe bind specifically to the respective DNA fragment. The quencher absorbs the fluorescence signal of the reporter dye. Step 2: DNA polymerase extends the primers. Step 3: DNA polymerase hydrolyses the probe. Step 4: by probe hydrolysis, the proximity of reporter dye and quencher dye is broken up. Step 5: reporter dye emits the fluorescence signal. Step 6: the more DNA is amplified, the more fluorescence signal can be detected
In order to quantify the content of GMO in a sample, a plant specific DNA fragment is quantified next to a GMO specific DNA fragment. The amount of GMO specific DNA copies is correlated to the amount of plant specific number of copies resulting in certain GMO contents [%].
Information on DNA sequence and the availability of certified reference material are essential before developing specific detection methods. For most of the authorised GM crops in the EU, reference material is available. However, this is not the case for non-authorised GMO.
When analysing a sample as part of the official food / feed / seed surveillance, a detection cascade is used as depicted in Figure 7. First, genetic elements commonly used in GMO are detected, which is not very specific. Very prominent genetic elements in GMO are the p35s promoter from the cauliflower mosaic virus (CaMV) or the tnos terminator from Agrobacterium tumefaciens. Methods detecting single genetic elements are named screening methods. By using screening methods, one cannot distinguish between different GMO, as many GMO contain common genetic elements. You can only generally detect a genetic modification. In order to gain specificity, construct specific detection methods are applied. By using these methods, a combination of two genetic elements (construct) is detected, which is much more specific than a screening.
A distinct identification of a GM crop can only be achieved by applying an event specific detection method. An event specific detection method is used to detect the intersection between the transgene and the plant genome. As the integration site is specific for each GM event, this is the most specific detection method for GMO. However, detailed sequence information is required to develop an event specific method.
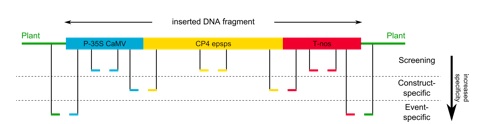
Figure 7: Detection cascade for the analysis of genetically modified crops
Evaluation of the results is facilitated by using a GMO database like the “GMOfinder”, which was developed at the Bavarian Health and Food Safety Authority (Gerdes et al. 2012).
Challenges to GMO Surveillance
In recent years, development of GM crops has been forced in developing countries, especially in Asia. Though these newly developed plants have been originally produced for the countries own market, it can not be ruled out that these products may also enter the EU market. Sequence information and reference material are missing for those events, which complicates the development of new detection methods.
By way of routine analyses in 2004, the Bavarian Health and Food Safety Authority detected genetic modification in papaya (Busch et al., 2004). GM rice can increasingly more often be detected (Babekova et al. 2009; Mäde et al. 2006), which shows that even though there are strict regulations and controls, products containing non-approved GM crops are exported to the EU.
In recent years, the development and planting of so called “stacked events” increased significantly. Stacked events are produced by cross-breeding of two or more gm crops. This leads to a new event featuring all traits of the original events. Stacked events also need to be authorized in the EU, as any other GM crop. This is a big challenge for GMO surveillance authorities, as you cannot distinguish between two single events or stacked events in complex matrices like food products.
An efficient, time and cost saving analysis workflow needs to be on hand in order to cope with the increasing use of gm crops worldwide.
References
Babekova, R, Funk, T, Pecoraro, S, Engel, K-H and Busch, U (2009) Development of an event-specific Real-time PCR detection method for the transgenic Bt rice line KMD1. Eur Food Res Technol. 228: 707-716.
Busch, U, Pecoraro, S, Posthoff, K and S. Estendorfer-Rinner, S (2004) Erster Nachweis einer gentechnisch veränderten Papaya in Europa - Beanstandung eines in der EU nicht zugelassenen gentechnisch veränderten Organismus. Deut Lebensm-Rundsch 100: 377-380.
EC (2003a) Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Offic. J. Europ. Union L 268/1.
EC (2003b) Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC. Offic. J. Europ. Union L 268/24.
Gerdes, L, Busch, U and Pecoraro, S. (2012) GMOfinder - A GMO Screening Database. Food Anal. Methods 5: 1368-1376.
Guertler, P. et al. 2013. Development of a CTAB buffer-based automated gDNA extraction method for the surveillance of GMO in seed. Eur Food Res Technol 236: 599-606.
Guertler, P, Paul, V, Albrecht, C and Meyer, HH (2009) Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Anal Bioanal Chem 393: 1629-1638.
James, C (2012) Global Status of Commercialized Biotech/GM Crops: 2012. ISAAA Brief No. 44: ISAAA: Ithaca, NY.
Mäde, D, Degner, C and Grohmann, L (2006) Detection of genetically modified rice: a construct-specific real-time PCR method based on DNA sequences from transgenic Bt rice. Eur Food Res Technol 224: 271-278.
Paul, V, Steinke, K and Meyer, HDD (2008) Development and validation of a sensitive enzyme immunoassay for surveillance of Cry1Ab toxin in bovine blood plasma of cows fed Bt-maize (MON810). Anal Chim Acta 607: 106-113.
Dr Patrick Guertler (e-mail: patrick.guertler@wzw.tum.de) and Dr Ulrich Busch (e-mail: ulrich.busch@lgl.bayern.ge) are with the Bavarian Health and Food Safety Authority, Molecular Biology Unit, Veterinärstr. 2, 85764 Oberschleißheim, Germany.
IUFoST Scientific Information Bulletin (SIB)
FOOD FRAUD PREVENTION