Don Mercer
Microwave ovens have become so commonplace that we hardly ever seem to give them a second thought. We use them to cook our food and reheat leftovers; or perhaps to reheat a cup of coffee or tea. The interesting thing about microwave ovens is how they actually heat the food.
To understand how microwave ovens operate, we really need to start by looking at the key player in all this – water. Most of the fruit and vegetables we consume contain over 80% water by weight. Even potatoes which are a slight exception contain about 78% water. Let’s begin with a brief description of the molecular structure of water in order to explain things.
As you probably know, water molecules are made up of two hydrogen atoms bonded to a single oxygen atom. Structurally, the hydrogen atoms are positioned on one side of the oxygen atom. Two lone pairs of electrons that are not bonded to anything are on the opposite side of the oxygen atom. While there is no overall charge on the water molecule itself, the side where the hydrogen atoms are located has a slight localised positive charge due to the presence of the proton in each hydrogen atom’s nucleus. Similarly, there is a slight localised negative charge on the side of the oxygen atom where the two lone pairs of electrons are situated. This makes the water a “polar” molecule which behaves like a rather weak magnet with a positive end and a negative end.
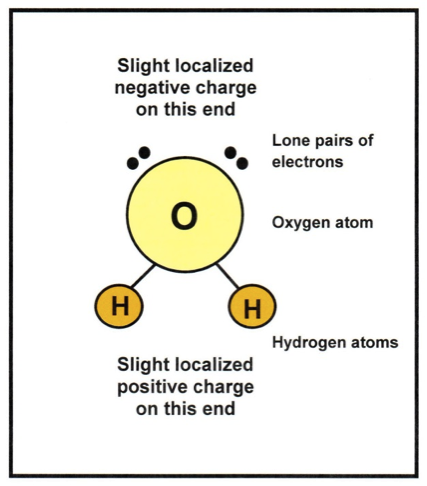
Diagram of a water molecule showing localised charges.
A magnet placed in an electromagnetic field will align itself so that its positive end points to the negative direction of the magnetic field. This is due to the fact that opposites attract. If we change the direction of the magnetic field, the magnet will turn to maintain its orientation with the positive end attracted to the negative direction of the magnetic field. Rapidly changing the direction of the magnetic field will make the magnet move back and forward at a greater speed.
Now, suppose we do the same thing to water by placing it inside a chamber where we can rapidly change the direction of the magnetic field. This will cause the water molecules to flip-flop back and forward as they try to maintain their proper alignment. In the process of moving at such high rates, the water molecules rub against each other creating friction at the molecular level, which in turn generates heat. The chamber to which we are referring here is actually the microwave oven. A magnetron inside the unit supplies the electromagnetic field that gets the water molecules moving.
Getting all the water molecules in motion doesn’t happen as soon as you press the microwave’s power button to turn it on. A bit of time is needed to get the water molecules stirred up from their normal state of rest.
Some of you may have noticed that if you microwave something for 60 seconds it will be hotter than if you heat it for 30 seconds, and then heat it again for another 30 seconds. Even though both cases involve the microwave being on for 60 seconds, the two 30 second cycles require two periods of getting the water molecules moving rather than only one time for the 60 second heating.
You may also have noticed that microwave ovens are a bit slower than we would like when it comes to defrosting foods. This is because water molecules in ice are organised in a tightly structured crystal arrangement that severely restricts their mobility, making it very difficult for them to move. Once some of the ice begins to thaw, the molecules in the liquid water that is present will have enough mobility to oscillate back and forth in the magnetic field and heat the material.
Microwave ovens tend to heat foods fairly uniformly. However, since microwaves penetrate only about one centimetre below the surface, there can be hot and cold spots in the material being heated. Turntables inside microwave ovens improve the evenness of heating. Stirring of liquids is another way to promote the distribution of heat. For solids, it may be necessary to simply allow time for the heat to spread. This is just one example of how modern food processing technology exploits the properties of materials at the molecular level.
Dr Don Mercer is Associate Professor in Food Science, Department of Food Science, University of Guelph, Guelph, Ontario N1G 2W1, Canada; e-mail: dmercer@uoguelph.ca
Permission to reproduce this article is greatly appreciated and acknowledged.
IUFoST Scientific Information Bulletin (SIB)
FOOD FRAUD PREVENTION