Gutenberg Delfino de Souza, Marcia Almeida de Melo, Éderson Akio Kido and Paulo Paes de Andrade
Abstract
Modern biotechnology can significantly contribute to many production areas. However, over regulation is hindering its adoption, especially in agriculture. Brazil has been well succeeded in both regulating and adopting agricultural biotechnology. A new legal framework, allowing the scientific assessment of GMO risks and clearly separating this step of risk analysis from those involving socio-economic considerations, was central to avoid authority conflicts and to offer GMO developers a safe legal scenario. Brazil has now one bean, five soybean, 12 cotton and 19 maize commercially approved GM varieties and 36.5 Mha of transgenic plants, being the second world leader in GM crops.
Introduction
Over the past 40 years Brazilian agriculture has considerably advanced thanks to the modernisation of its production sector, allowing the country to meet the domestic market demand and to export surplus production. Such a scenario was only possible due to the scientific and technical advances in agriculture that, besides improving productivity, also added new value, safety and quality to its products (http://www.harvestplus.org/content/biofortification-move-forward-lac). It is unquestionable that the agricultural sector has been steadily modernised (for both small and large producers), being now responsible for the economic sustainability of the country (Serigati 2013).
Brazil, as one of the three largest agricultural producers in the world, seeks to reconcile scientific advances in agribusiness to environmental responsibility. Biotechnology has undoubtedly contributed to this binomial, especially in the responsible use of resources (water, fuel, pesticides) and in the search for new products that will contribute to the agricultural environmental sustainability and the productive sector as a whole (plant varieties resistant to biotic / abiotic stresses or biofortified). The adoption of biotechnology products in agriculture decisively helped Brazil reach this production level (Céleres 2012), even with the late adoption of genetically modified (GM) crops, due to the legal uncertainties that permeated the Brazilian legal scenario from 1998 to 2005. The use of GM plants increases each year, thanks to the diversity of varieties available in the market, and to a suitable legal framework, essential for the stability of any economic sector (Sousa and Andrade 2012). Profiting from these exceptional conditions, Brazil has reached the second position worldwide in cultivated GM crop area (Figure 1).
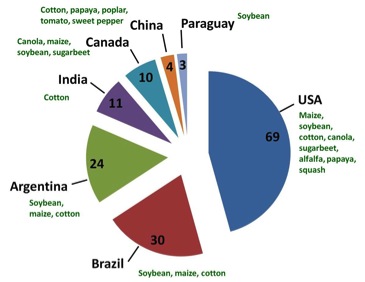
Figure 1: Brazil holds the second position in the World rank of GM crops producers. Adapted from ISAAA Brief44-2012 (http://www.isaaa.org/).
The main GM agronomic products grown in Brazil are soybeans, corn and cotton, all belonging to the first generation of transgenic plants displaying insect resistance and / or herbicide tolerance. Brazil has also its own product of the utmost importance for the food industry: a virus-resistant common bean (Phaseolus), developed by EMBRAPA; this is the first GM plant at the stage of marketing and fully developed by a Brazilian company. Other agricultural products under research include eucalyptus (increased volume of wood), wheat (abiotic stress), soybean (improvement in oil quality, resistance to Asian rust), citrus (disease resistance) and rice (increased productivity).
Apart from GM plants 18 recombinant vaccines and two yeasts (transformed to oil production from sucrose and other fermentable sugars) have been approved for the Brazilian market. The majority of those recombinant vaccines was developed for poultry and swine, so that also contributes to the increased supply of food of animal origin and to the improvement of its quality, with clear benefits for both the domestic market and exports.
The Brazilian legal framework
Until 2005 Brazil had a rather confusing legal scenario for both research activities and the commercial release of GMOs (Capalbo et al. 2003, Fontes 2003). Back in 1998, the commercial approval of Roundup Ready® soybeans by the National Biosafety Technical Commission (CTNBio) evoked a long and sterile debate for more than 6 years, centered on non-scientific issues or, sometimes, on misquoted scientific data. Moreover, other purely scientific activities, as laboratory experiments and field trials, were largely prohibited, until a new law was issued on March 2005 restructuring the CTNBio and providing for many other issues and, ultimately, authorising the first harvest of the transgenic soybean
(http://www.ctnbio.gov.br/index.php/content/view/12847.html).
The new law dramatically changed the scenario (Naves and Freire de Sá 2005, Tanus Job 2008); it provided for safety norms and inspection mechanisms for the construction, culture, production, manipulation, transportation, transfer, import, export, storage, research, marketing, environmental release and disposal of genetically modified organisms (GMOs) and their by-products, among other measures (see Box 1). It also eliminated the many authority conflicts which plagued the first law and were on the basis of a legal uncertainty that simultaneously drove off the biotech companies and allowed for endless legal questionings related to both GMO field experiments and commercial releases.
Main features of the Brazilian Biosafety Law
Law 11105 / 2005 (Brazilian Biosafety Law)
Creates the National Biosafety Technical Commission – CTNBio ̶ as a consultative and deliberative body for all activities related to the use of genetic engineering techniques and products derived from this technology
Establishes safety standards and enforcement mechanisms for the activities with GMOs and their derivatives
Fosters scientific advances in the area of biosafety and biotechnology
Ensures the protection of the environment and of animal, plant and human health
Fosters the observance of the precautionary principle to protect the environment and human health
The law also created the Internal Biosafety Committees (CIBio), which act as managers and supervisors of any activity with GMOs within institutions that develop any work in modern biotechnology (research, development, innovation or production). On a higher level the law established the responsibilities for supervision of uncontained activities with GMOs and registration of new products, making the Ministry of Agriculture and Livestock (MAPA), the National Agency for Sanitary Surveillance (ANVISA) and the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) responsible for these activities. The law also created at the top level the National Biosafety Council (CNBS), which analyses the eventual socioeconomic impacts of GMOs, and may revoke a CTNBio decision to commercially release a new biotechnology product following its risk assessment. The relationship between these actors in the Brazilian GMO regulatory scenario is shown in Figure 2.
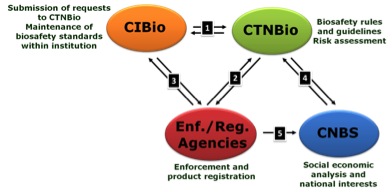
Figure 2: Main players in the Brazilian biosafety regulatory scenario. More than 300 Biosafety Internal Commissions (CIBio) warrant GMO biosafety within private and public institutions:
[1] They submit reports, questions and requests for commercial release of a new GMO to the National Biosafety Technical Commission (CTNBio) which, in return, evaluates annual reports and the requests and gives specific instructions on biosafety. CTNBio also issues the Biosafety Quality Certificate, essential to carry out any work with GMOs; [2] Once a risk assessment is completed for a request either for field or commercial release of a new GMO, CTNBio communicates its decision to the Enforcement and Registration agencies (on health, environment and agricultural issues), which report back to CTNBio any non conformity; [3] The Enforcement agencies also require compliance with specific measures for biosafety to the CIBio which, in return, report their activities to the agencies; [4] CTNBio reports to the National Biosafety Council (CNBS), composed of 11 Ministers of State, the results of its risk assessment and other decisions and helps the Council to define the National Biosafety Policy. The Council, in return, issues broad regulatory norms on biosafety; [5] Finally, the Enforcement agencies can appeal to the CNBS to revoke a CTNBio decision regarding commercial release.
Moreover, each institution working with a GM organism must possess a Quality Certificate in Biosafety (CQB) as well as an Internal Biosafety Commission. Brazil has currently 353 institutions holding a CQB. All this regulatory setting is intended to give functionality to institutions that operate in the genetic engineering field in Brazil, simultaneously avoiding conflicts.
The National Technical Biosafety Commission, centerpiece of the Brazilian biosafety System, is a subsidiary body to the Ministry of Science, Technology and Innovation, and had its first configuration sponsored by the Law nr. 8974 / 95. Currently its Board, composed of 27 members and their alternates, includes experts of widely recognised scientific knowledge, indicated by scientific societies and the academic community in the areas of human and animal, plant and environmental health, and representatives from nine Ministries (Box 2). Experts in consumer protection, health, environment, biotechnology, agriculture, small family farms and worker health have sit on the Committee. Additionally, CTNBio has a Secretariat and a permanent technical body assisting the Board and having other regular activities.
Composition of the Brazilian National Biosafety Technical Commission (CTNBio) Board
12 specialists of recognised scientific and technical knowledge in the areas of human, animal, plant and environmental
9 ministerial representatives:
MCT (Science and Technology)
MAPA (Agriculture and Livestock)
MS (Health)
MMA (Environment)
MDA (Agricultural Development) MDIC (Industry and Commerce)
MD (Defense)
MAP (Fisheries)
MRE (Foreign Affairs)
6 specialists: Consumer protection (Ministry of Justice), health (MS), environment (MMA), biotechnology (MAP), family farming (MDA) and worker health (Ministry of Labor)
Total: 27 members and 27 substitutes; Monthly meetings
The main function of CTNBio is to assess the risk of a GMO, i.e the genetic transformation biological implications, derived either from research activities, or large scale production in containment, experimental or commercial releases. Socioeconomic considerations, embedded in the risk analysis (Andrade et al. 2012) are the sole responsibility of the National Biosafety Council - CNBS, composed of eleven Ministers of State. The CNBS opinion can only be invoked in the commercial phase of the use of a GMO; the Council is responsible in the last and final instance for the decision to introduce GMOs on the market.
CTNBio is divided into four sectoral chambers or subcommittees operating within their area of expertise (human, animal, plant and environment). Applicants´ requirements to CTNBo are distributed to at least two Sub-commissions, encompassing two chambers (human/animal health or plant health/environment) and delegated to a small number of members appointed in each case. Their opinions are subsequently discussed among the other members, the Sub-commission position of each process is preliminarily voted and finally forwarded for the discussion by all members of the Board in Plenary sessions. However, each member has the right to request a copy of any process on the CTNBio agenda for his / her own analysis, Final decisions are reached by voting (Figure 3).
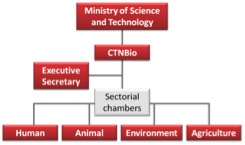
Figure 3: CTNBio structure
Approval of GM Crop Varieties and Adoption of Agricultural Biotechnology in Brazil
The force of Brazilian agriculture, denoted by an extensive list of exports, was already visible before the adoption of GM plants. The association between an efficient national agriculture and the technological advance represented by GM plants, a synergy that could only bring benefits to the country, was only possible due to the Brazilian regulatory clarity. Since the enactment of the new law on biosafety, dozens of GMOs have been approved for commercial use, especially GM plants. The varieties quickly gained the market and are now planted in all areas of traditional cultivation of cotton, corn and soybeans. Table 1 shows the transgenic crop varieties approved by CTNBio and the year in which they were approved. These plants have two phenotypes, showing up separately or together: insect resistance or herbicide tolerance.
Table 1. Genetically modified crop varieties approved for commercial use in Brazil. Source: www.ctnbio.gov.br – accessed 06/26/2013
|
Crop |
Phenotype |
Protein |
Year of approval |
|
Soybean |
Herbicide tolerant |
CP4-EPSPS |
1998 |
|
Herbicide tolerant |
Csr-1-2 |
2009 |
|
|
Herbicide tolerant |
PAT |
2010 |
|
|
Herbicide tolerant |
PAT |
2010 |
|
|
Herbicide tolerant & insect resistant |
CP4-EPSPS Cry1Ac |
2010 |
|
|
Maize |
Insect resistant |
Cry1Ab |
2007 |
|
Herbicide tolerant |
PAT |
2007 |
|
|
Insect resistant& herbicide tolerant |
Cry1Ab PAT |
2007 |
|
|
Herbicide tolerant |
CP4-EPSPS |
2008 |
|
|
Herbicide tolerant |
mEPSPS |
2008 |
|
|
Insect resistant & herbicide tolerant |
Cry1F PAT |
2008 |
|
|
Herbicide tolerant & insect resistant |
CP4-EPSPS Cry1Ab |
2009 |
|
|
Herbicide tolerant & insect resistant |
Cry1Ab PAT mEPSPS |
2009 |
|
|
Insect resistant |
VIP3Aa20 |
2009 |
|
|
Insect resistant & herbicide tolerant |
Cry1F PAT CP4-EPSPS |
2009 |
|
|
Insect resistant |
Cry1A.105 Cry2Ab2 |
2009 |
|
|
Insect resistant & herbicide tolerant |
Cry1Ab VIP3Aa20 mEPSPS |
2010 |
|
|
Insect resistant & herbicide tolerant |
Cry1A.105 Cry2Ab2 CP4-EPSPS |
2010 |
|
|
Herbicide tolerant & insect resistant |
CP4-EPSPS Cry3Bb1 |
2010 |
|
|
Insect resistant & herbicide tolerant |
Cry1A.105 Cry2Ab2 Cry1F PAT CP4-EPSPS |
2010 |
|
|
Herbicide tolerant & insect resistant |
cry1Ab Cry1F PAT CP4EPSPS |
2011 |
|
|
Herbicide tolerant & insect resistant |
Cry1F Cry1Ab PAT |
2011 |
|
|
Herbicide tolerant & insect resistant |
Cry1A.105 Cry2Ab2 Cry3Bb1 CP4-EPSPS |
2011 |
|
|
|
Herbicide tolerant & insect resistant |
Cry1F PAT Cry34Ab1/cry35Ab1 |
2013 |
Table 1 (ctn)
|
Crop |
Phenotype |
Protein |
Year of approval |
|
|
Cotton |
Insect resistant |
Cry1Ac |
2005 |
|
|
Herbicide tolerant |
CP4-EPSPS |
2008 |
||
|
Herbicide tolerant |
PAT |
2008 |
||
|
Herbicide tolerant & insect resistant |
Cry1Ac CP4-EPSPS |
2009 |
||
|
Herbicide tolerant & insect resistant |
Cry1Ac Cry1F PAT |
2009 |
||
|
Insect resistant |
Cry2Ab2 Cry1Ac |
2009 |
||
|
Herbicide tolerant |
2mEPSPS |
2010 |
||
|
Insect resistant & herbicide tolerant |
Cry1Ab Cry2Ae PAT |
2011 |
||
|
Herbicide tolerant |
CP4-EPSPS |
2011 |
||
|
Herbicide tolerant & insect resistant |
Cry1Ab, cry2Ae,2mepsps |
2012 |
||
|
Herbicide tolerant |
2mepsps, bar |
2012 |
||
|
Herbicide tolerant & iInsect resistant |
cry1Ac & cry2Ab2 & CP4-EPSPS |
2012 |
||
|
Bean |
Resistant to the Bean Golden Mosaic Virus (BGMV) |
No new protein is produced, only siRNA |
2011 |
|
Once a variety is considered safe as food / feed and for the environment by CTNBio, it must also be recognised as an adequate crop variety for one or more Brazilian agricultural areas. The process of certification, submitted to and analysed by the Ministry of Agriculture and Livestock, usually starts well before the final CTNBio opinion, allowing the immediate commercialisation of a recently approved GM crop variety. Figure 4 depicts the rapid and steady increase on GM crop adoption in Brazil.

Figure 4: Adoption of GM crops in Brazil in terms of percentage of available crop area planted with GM varieties. Total area for the three crops equals 36.5 mi. ha. (Adapted from Celeres 2012a)
Agricultural biotechnology clearly brought benefits to farmers from traditional producing areas of cotton, corn and soybeans (Figure 5). The adoption rate and huge areas planted with GM varieties (Céleres 2012a) are the best proof of this statement, since the purchase of a particular seed variety is always a decision of the farmer, determined by the market and not an imposition of any government program. The reduction of pesticide use, irrigation water and fuel for farm machinery, associated with productivity gains and reduction of losses, implied a significant reduction in costs for the farmer (Céleres 2012b). The estimated benefits were US$ 18.8 B from 1997 to 2012.
From the environmental point of view the adoption of biotechnology in Brazil also brought benefits, with reduced use of pesticides, water, machinery and fuel, and reducing the rate of deforestation due to better land use. There was also a reduction in CO2 emissions, if current rates are compared to those predicted for a 100% conventional agriculture (Céleres, 2013).

Figure 5: Illustration of the adoption rates of GM cotton, corn and soybean in several Brazilian states. The circles are proportional to the adoption of GM technology. The yellow circles correspond to 98% adoption.
Conclusion
Despite the massive adoption of GM plant varieties by farmers in Brazil, no impacts were observed in the wild or agricultural environments that could be specifically attributed to GMOs. There was no gene flow and establishment of transgenes in native cotton species or landraces of maize. Even the question of coexistence between conventional and transgenic technologies seems to be resolved through the use of coexistence rules based on agronomic practice and science. The absence of appeals to the courts for compensation for losses due to conventional grain contamination with GM varieties supports this statement. On the other hand, there were clear environmental benefits, represented mainly by the reduction on the use of machines, irrigation water and pesticides.
Thus, despite the fact that the Brazilian regulatory system is focused on regulating the technology and not the product, the end result has been positive. Looking ahead, however, this approach can be harmful, especially with the increasing demonstration that this technology does not bring additional risks to the environment or to human or animal health than those possibly coming from other conventional techniques used to generate and select new genetically improved varieties of plants, microorganisms and viruses. In addition, new technologies are emerging in the market and it makes no sense to regulate each one of them, but just their products.
The Brazilian regulatory costs are high for both the Government (CTNBio maintenance and specific actions of the registration and inspection agencies) and for the private sector. Especially for the latter, the costs of marketing approval of new GMOs can surpass five million dollars. These high costs are in clear disproportion to the observed risks and hinder the access of small companies to the domestic market of modern biotechnology as well as discourages research within and outside the universities. The post-commercial release regulatory costs are still small, since the new system of GMO monitoring allows for low cost effective measures (http://www.ctnbio.gov.br/index.php/content/view/18000.html; Melo et al. 2011; http://genpeace.blogspot.mx/2011/12/brazils-new-post-release-monitoring.html). An overall reduction of costs will depend on the change of Normative Resolution 5, which now requires companies wishing the commercial release of a GMO to respond with experimental data to a long list of questions that are largely not related to the risk assessment of the specific GMO and which date back to a time where the uncertainties about the technology were very large.
Finally, despite its cost and its apparent complexity, the Brazilian biosafety system works well and allowed the adoption of GM technology without impacts on the environment and human and animal health. This is due in large part to the absence of conflicts of authority, since the decision to release a GMO is basically technical and performed by a single body, specially created for this purpose. All socioeconomic issues are discussed, where appropriate, by the National Biosafety Committee. Thus, it is natural that only issues of great relevance to the agenda of discussions come to the CNBS. The task of accepting or rejecting the technology and resolving any conflicts of use is effectively delegated to the market.
Although it works, the Brazilian model should not be copied without important changes by countries wishing to adopt GM technology, as it regulates all GMOs (not just plants or animals) and because it is very costly. However, the clear separation of responsibilities, which prevents conflicts, and especially the separation of risk assessment and the remaining steps of the risk analysis is a feature that should be sought by any legislator who wishes to lead their country to adopt modern biotechnology in agriculture and in other production areas.
Bibliography
Andrade, PP, Parott, W and Roca, MM (2012) Guía para evaluación de riesgo ambiental de organismos genéticamente modificados. International Life Sciences Institute of Brasil, São Paulo, Brazil; 140 pp.
Capalbo, DM, Hilbeck, A, Andow, D, Snow, A, Babong, B, Wan, FH, Fontes, EM, Onyango Osir, E, Fitt, GP, Johnston, J, Songa, J, Heong, KL and Birch, AN (2003) Brazil and the development of international scientific biosafety testing guidelines for transgenic crops. J. Invertebr. Pathol. 83(2):104-6.
Céleres (2012a) Relatório de Biotecnologia, 6 de agosto de 2012. Available at: http://celeres.com.br/wordpress/wp-content/uploads/2012/12/RelBiotecBrasil_1201_vf.pdf. Accessed in 06/26/2013 .
Céleres (2012b) The commercial benefits from biotechnology in Brazil:1996/97 to 2011/12. Available at http://www.celeresambiental.com.br/pdf/PressRelease2011_Economico_Eng.pdf. Accessed in 06/29/2013
Celeres (2013) Benefícios socioambientais da biotecnologia no Brasil: 1996/97 a 2011/12. Avaliable at: http://celeres.com.br/wordpress/wp-content/uploads/2013/01/PressRelease2012_Ambiental.pdf. Accessed in 06/29/2013
Fontes, EMG (2003). Legal and regulatory concerns about transgenic plants in Brazil. J. Invert. Pathol. 83(2): 100–103.
Melo, MA, Kido, EA and Andrade, PP (2011) Post market monitoring: Legal framework in Brazil and first results. J. Cultivated Plants 63: 226-231.
Naves, BT, Freire de Sa, MF (2005) Biotecnología y aspectos relevantes de la nueva Ley de Bioseguridad. Law. Hum. Genome. Rev. 22: 85-102.
Serigati, FA (2013) Agricultura puxa o PIB? Agroanálysis. Fev.13-14.
Sousa, GD and Andrade, PP (2012). Ambiente Regulatório de Organismos Geneticamente Modificados no Brasil . In: Diversidade e Inovações na cadeia produtiva de milho e sorgo na era dos transgênicos. IAC: 139-159.
Tanus Job, BM (2008) The previous risk treatment of the transgenic crops in the Brazilian biosafety regulatory system. Law. Hum. Genome. 28:159-74.
Gutenberg Delfino de Souza is with the Brazilian National Biosafety Commission (CTNBio), Brasilia, DF, Brazil; Marcia Almeida de Melo is with the Unit of Veterinary Parasitology, Federal University of Campina Grande, Patos, PB, Brazil; and Éderson Akio Kido and Paulo Paes de Andrade (corresponding author) are with the Department of Genetics, Federal University of Pernambuco, Recife, PE, Brazil; E-mail: andrade@ufpe.br
IUFoST Scientific Information Bulletin (SIB)
FOOD FRAUD PREVENTION